Liver Cancer Biomarker

Tech ID
P35-1002
Patent Status
Pending
Design Status
---
Technical Description
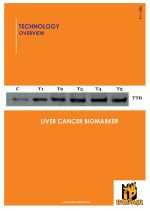
A diagnostic kit capable of early diagnosis of a particular protein biomarker and molecules, binding to said biomarker, for early detection of Hepatocellular carcinoma.
The present invention involves analyzing proteins expressed and differentially expressed during liver cancer progression and assess their potential for the development of biomarkers for early detection of HCC. Using the procedure of two-dimensional gel electrophoresis, a subset of proteins that appear to distinguish between the control and treated subject in a statistically significant manner has been detected.
ADVANTAGES
The kit has utility in many areas, including the following:
- Screening individuals, at an increased risk for liver cancer;
- Establishing the specific liver cancer sub-type at the time of diagnosis;
- Providing an indication of prognosis for individuals diagnosed with a specific liver cancer sub-type; and
- Providing novel approaches for therapy, based on understanding of the role of these proteins in different liver cancer sub-types.
Areas of Application
Diagnostic Kits andDevices
P11-1002
Send download link to email.
Available For
Exclusive and NonExclusive License Sell off in Indian Territory
Cost
On Request
Stage of Developments
Feasibility test completed with astonishing results.
Current Market
Identification of early HCC and improve survival is the rationale behind screening for HCC. An effective screening program, however, requires certain criteria an identifiable target group, acceptable tests with high sensitivity and specificity, and available treatment.
Surveillance intervals forHCCare based on a balance between the tumor doubling time and the cost of the screening tests. Doubling time of HCC ranges from 1 to 19 months with a median of 4–6 months. Most study protocols conduct screening every 6 months. The overall cost of surveillance for HCC varies according to region, population incidence, and the screening tools used. The cost of finding each tumor in high-risk individuals ranges from $ 11,000 to $25,000.
Therefore, there is a huge market for novel diagnostic kits and devices.
Contact Person

Ms. Shruti Kaushik
IP Bazzaar Technology Pvt. Ltd.
New Delhi-110024, India
Tel: [+91] 11-40110403;
Fax: [+91] 11-26360037;
Mobile: [+91] 9810338816
Email: tech@ipbazzaar.com;
www.ipbazzaar.com
